Technology
InkVivo Technologies has developed a proprietary polymer-based platform designed to transform drug development and delivery. Built on FDA-approved and GRAS materials, our system enables controlled, localized, and sustained release of active ingredients across multiple administration routes. This innovation combines modular polymer design, advanced processing, and data-driven optimization to deliver superior therapeutic performance and industrial scalability.
The science behind InkVivo
InkVivo’s technology is built on over six years of combined academic and industrial research, originating from ETH Zurich. The platform leverages a multi-structural polymer system designed for precision in drug delivery.
At its core
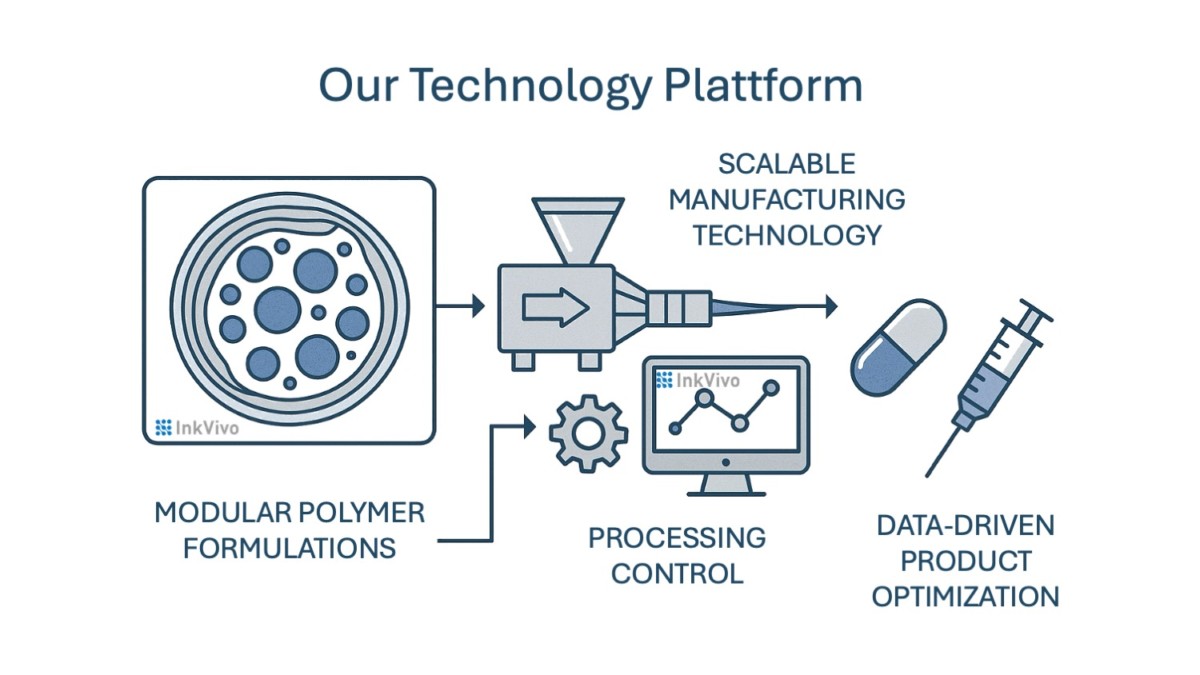
Scientific Rationale InkVivo’s technology combines advanced encapsulation approaches, composite structures, and controlled release mechanisms (e.g., erosion, diffusion).
Modular Polymer Library InkVivo’s proprietary formulations encapsulate a wide range of active ingredients (e.g., small molecules, biologics, and micronutrients) while maintaining stability in harsh environments such as gastric conditions.
Tailored Release Kinetics Through engineered physical interactions and spatial design, each active ingredient can be released with its own controlled profile ensuring prolonged absorption, increased therapeutic efficacy, and minimized side effects.
Biocompatibility and Safety All polymers are FDA-approved or classified as GRAS, ensuring compliance with international standards.
What makes it unique
Unlike conventional systems, InkVivo integrates data-driven optimization and advanced formulations to accelerate development cycles while relying on scalable manufacturing approaches. This enables rapid iteration from concept to prototype in a few weeks, reducing R&D costs and time-to-market.
Advanced Formulations and Scalable Manufacturing
InkVivo’s proprietary processing techniques allow precise control over spatial composition and release profiles. The manufacturing approach implemented by InkVivo can be industrialized and support scale production without compromising quality or stability of active ingredients. This approach ensures cost-efficiency and future compliance with GMP standards.
Protected Innovation
Our platform is secured by granted patents in Europe and China, with additional filings covering gastro-retentive formulations. This strong IP portfolio ensures freedom to operate and long-term value for our partners.
Data-Driven Optimization
We integrate computational modeling and AI-assisted design to optimize polymer formulations and release kinetics. This data-driven approach reduces development time from months to weeks, enabling rapid adaptation to partner needs and regulatory requirements.
Built for Regulatory and Environmental Standards
All materials and processes comply with FDA and EMA guidelines. Our technology minimizes resource use and could be implemented in decentralized manufacturing, reducing environmental impact while maintaining strict quality standards.
